A late-stage trial of Eli Lilly’s inhaled insulin is slated to wrap in time for the firm to submit the product in 2009, an executive said today.
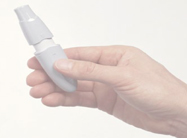
Lilly’s inhalable insulin device fits in the hand and is easy for patients to use, said Bryce Carmine, Lilly global brand director.
That could help the investigational product steal a march on Pfizer, whose Exubera inhaled insulin, launched last year, has not been well received. The product is estimated to have generated only about $5 million in sales last year, a fraction of some forecasts. One reason is the larger size of the delivery device. Another: concerns among diabetes specialists about the long-term effects of Exubera on the lungs.
“We continue to be encouraged by the safety of inhaled insulin,” Carmine said at the 2007 UBS Global Life Sciences Conference. Lilly plans US and EU filings in 2009.
The pulmonary drug delivery system for diabetes, based on a technology called “Air” that Lilly is co-developing with Alkermes, was just one of six late-stage products he highlighted, in addition to listing about 15 early stage candidates.
Prasugrel, a blood thinner for use in acute coronary syndrome, is set to be submitted to the FDA by year’s end. Some analysts view prasugrel as the most anticipated potential approval for 2008.
Its clinical trials have been designed to demonstrate superiority over Plavix (clopidogrel), Bristol-Myers Squibb/Sanofi Aventis’s $5-billion-a-year seller which is set to go generic in 2012. (Plavix saw brief generic competition last year due to generics firm Apotex’s at-risk launch.) Lilly is scheduled to read phase III data on prasugrel in November at the American Heart Association meeting.
Also on track to emerge from Lilly’s pipeline near-term are a long-acting form of diabetes drug Byetta, administered through once-weekly injections, and an extended-release form of the antipsychotic Zyprexa. In addition, arzoxifene, a molecule with higher potency than Lilly’s Evista osteoporosis drug, has a scheduled 2009 filing date for treatment and prevention of osteoporosis as well as breast cancer risk reduction, while enzastaurin, a once-daily PKC-beta inhibitor for non-Hodgkins Lymphoma is slated for filing as soon as late 2010.
In light of the outlook for candidates in the firm’s pipeline, Carmine said, Lilly would aim for launching two new medicines a year beginning early in the next decade.
Meanwhile, early in the next decade is when Wyeth’s fortunes could take a less-than-favorable turn. In the 2010-2011 window, Wyeth is set to face generic competition on two of its biggest drugs, antidepressant Effexor and gastrointestinal treatment Protonix—two drugs which combined for about $1.5 billion in second-quarter sales.
For a year or two in that time frame, earnings will indeed be “choppy,” acknowledged Greg Norden, Wyeth CFO, at the UBS meeting.
He added that products like the firm’s autoimmune drug Enbrel, which it co-markets with Amgen, and the Prevnar vaccine will “grow through that period” due to additional launches and, in the case of Prevnar, increased market penetration. Wyeth hopes to gain approval for an expanded form of Prevnar, as well as market it for use among adults along with infants. Norden said the firm expects peak sales of $4.5 billion for the vaccine.
The CFO also cited his firm’s Alzheimer’s development program, yet-to-be-discovered in-licensing deals, early stage pipeline products and the consumer, nutritional and animal health units as reasons why 2010 could mark the start of a “very bright future for the company.”