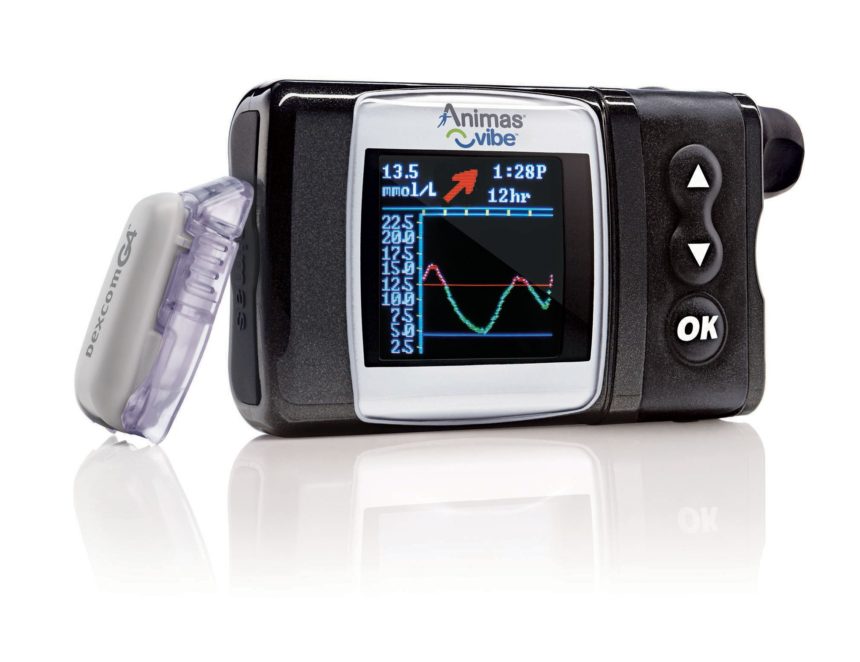
FDA approved the Animas Vibe insulin pump and continuous glucose monitoring (CGM) system on Monday, giving diabetes patients a second choice in insulin pumps.
In an FDA Consumer Update, describing patient choices in diabetes management, the agency saidthe Animas pump gives consumers more choices in the types of CGMs that can be integrated wirelessly with an insulin pump.
Johnson & Johnson Diabetes Solutions unit Animas announced approval of the system for management of insulin-requiring diabetes in adults, setting up a showdown with Medtronic’s MiniMed Paradigm Veo insulin pump, which has had the market to itself since 2006.
Medtronic reported $1.5 million fiscal 2013 sales in its diabetic segment driven by its MiniMed and Enlite continuous glucose monitoring sensor.
Animas Vibe allows patients to view glucose data as insulin is administered from the pump, measuring glucose levels every five minutes, providing real-time glucose values, and trend information as patients see their latest glucose readings on the pump screen.
Customizable alarms indicate high and low glucose levels helping patients fine tune the insulin delivery.
Users can also personalize the system’s precise dosing by choosing individual insulin-to-carb ratios, insulin sensitivity factors, and blood glucose targets in 30-minute increments with up to 12 personal settings.
The system features Dexcom G4 PLATINUM sensing technology, approved for up to seven days of continuous wear with one of the smallest introducer needles on the market. With Mean ARD (Absolute Relative Difference) as the industry’s standard for measuring CGM accuracy, Dexcom’s 13% Mean ARD provides exceptional performance and accuracy for reliable results, Animas reports.
The information available from the screen readouts complements fingerstick testing results, helping to guide patients in immediate and long-term insulin delivery therapy adjustments.
CGM has been shown to improve glycemic control in adult patients 25 years and older via lower HbA1c levels, less frequent hypoglycemia, and reduced glucose variability, Animas says.
Shipments will begin in 2015 for the system which is available now in Europe, Australia, New Zealand, and Canada.