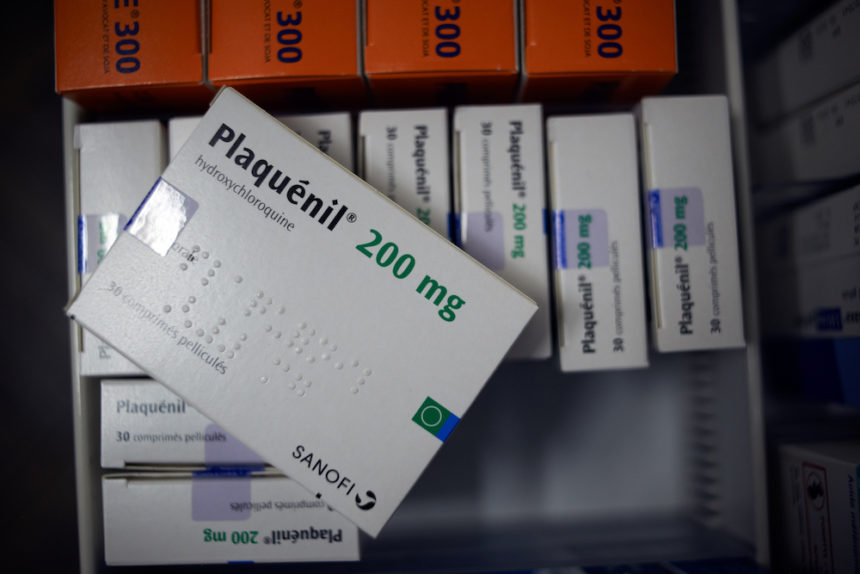
Malaria drug showed no benefit in coronavirus patients in one study. The study analyzed use of hydroxychloroquine in U.S. veteran’s hospitals. There were more deaths among those given hydroxychloroquine versus standard care, researchers reported. (Associated Press)
The first at-home COVID-19 test was approved by the Food and Drug Administration. The test, developed by LabCorp, allows people to collect a sample at home using a cotton swab in the nose. It will be made available first to healthcare workers and first responders. (STAT)
New York state has charged Mallinckrodt for misrepresenting safety of opioids. The company was charged with insurance fraud for misrepresenting the safety of its opioid drugs, leading to medically unnecessary prescriptions. (Reuters)
The leader of government agency Biomedical Advanced Research and Development Authority (BARDA) has left. Rick Bright moved to a smaller role with the National Institutes of Health. BARDA is the agency tasked with investing in treatments and vaccines for infectious diseases and has been at the center of the U.S. coronavirus response. (STAT)
Evidence suggested that the coronavirus originated in animals in China, and was not created in a lab, according to the World Health Organization. President Donald Trump said last week that the government was trying to determine if the virus was created in a lab. (Reuters)