Life changing — a pretty good way to describe 2020. For most, it will go down in the books as a year we will all want to quickly forget. But there was also a bright side, as far-reaching therapeutic innovations pushed the boundaries of what’s possible. To put this into context, during the first decade of the 21st century, the FDA approved an average of 23 novel drugs each year. In just the last two years alone, the number of approvals more than doubled. And 40% of the novel drugs approved were designated first-in-class. Without a doubt, this is a very exciting time to be working in the field of medical innovation.

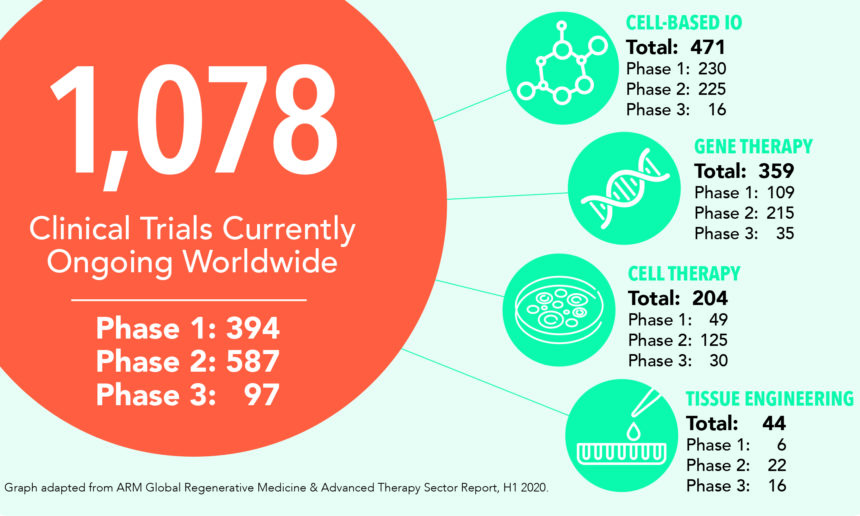
In 2020, the floodgates opened for these breakthrough therapies. Late-stage gene therapies have continued to demonstrate long-term durability. Patients with rare genetic disorders now have a targeted treatment, thanks to the translation of Nobel Prize-winning science into clinical therapeutics using RNA interference. The one-size-fits-all approach to treating cancer is quickly being replaced by personalized, tumor-directed cellular immunotherapies. And 2021 shows no signs that these advancements will slow down. Case in point, more than 1,000 clinical trials are ongoing in gene and cell therapies with close to 100 trials expected to enter the regulatory phase in the coming months. Moreover, the science is expanding to more complex and novel ways of treating familiar diseases, as well as new diseases or conditions. Brand new, first-of-their-kind advances that promise to transform patient care — these are therapies that we call lifechanging.
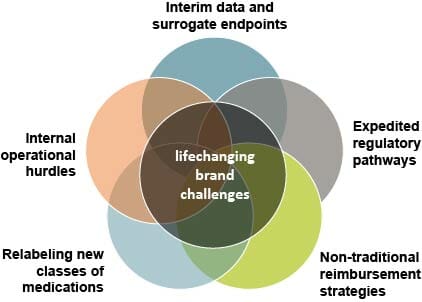
With the excitement of these advancements, we turn our thoughts to marketing strategy. Surely we can use our trusty marketing playbook to get the message across, right? Not always. We’re finding that lifechanging therapies present new strategic, clinical and operational challenges that need to be recognized and addressed, often with faster timelines, to stay ahead of the competition and to maximize impact. These are complexities that many emerging biotech companies are trying to figure out in real time, including how to leverage interim data and surrogate endpoints, ever-changing expedited regulatory pathways, non-traditional reimbursement strategies, relabeling new classes of medications and numerous internal operational hurdles. In addition, these companies sometimes need to launch their own corporate presence. In 2020, nearly one-third of novel drug approvals represented a first commercial launch for the sponsoring company, and 68% of all novel drugs were classified for an expedited review pathway. Knowing what to do when there isn’t a clear playbook can truly make or break a brand’s — and a company’s — success. An agile, creative agency partner that is adept at navigating these strategic, clinical and operational challenges is also required. And we at CDM Princeton are excited to be that agency.
Our sole focus is lifechanging brands — those therapies that promise to improve the world by satisfying urgent patient needs. Our impressive portfolio of first-of-their-kind life-changing brands includes groundbreaking gene therapies, novel biomarker-based plasma tests and the first-ever erythroid maturation agent, to name a few. With each new brand, new challenges had to be overcome, helping us to truly master the skills needed to build breakthrough brands in today’s new world of healthcare.
We’re looking forward to a 2021 (and beyond) full of life-changing brands to energize and inform us. So bring on the curveballs, tell us “we’ve never done this before,” come to us with emergency approvals and impossible timelines and we’ll show you that at CDM Princeton, we make lifechanging happen.
Here’s to an incredibly exciting year ahead!
From the February 01, 2021 Issue of MM+M - Medical Marketing and Media