Novartis’s Sandoz division is revving up marketing plans for its Neupogen knockoff as it waits to see if the FDA will approve its injectable cancer drug as the first biosimilar in the US. Obstacles loom, the first of which is American doctors’ lack of familiarity with biosimilars as well as pressure from future rivals and an expected counter-detailing push by innovator company Amgen.
Novartis is looking for an FDA decision as early as March, based on an FDA goal of reviewing 50% of biosimilar applications received in 2014 within 10 months, under a Biosimilar User Fee Act requirement, a Novartis spokesperson told MM&M.
While the drug Sandoz calls Zarxio won unanimous backing from an FDA panel in January as a “highly similar” version of Amgen’s filgrastim brand, with no clinically meaningful difference to the reference drug, prescribers still need to be convinced it works as well as Neupogen, a drug with which they have more than two decades of familiarity. Launched in 1991, the drug posted 2013 US sales of $963 million.
“[Sandoz] will support Zarxio primarily through contracting (with insurers) and with a small Sandoz sales and marketing force of about 50 people,” Bernstein analyst Ronny Gal said.
As a marketer experienced in biosimilars, with three such products on the market in Europe, including a filgrastim it sells under the name Zarzio, Sandoz will be educating US doctors on the biosimilars concept as well as detailing the comparability and cost advantages of Zarxio vs. alternative G-CSFs.
Helping to make the case with doctors, Sandoz detailers will be equipped with postmarketing data derived from the drug’s use in more than 40 countries, leading to 7.5 million patient exposure days of experience.
Sandoz plans to roll out two rounds of marketing to doctors, Gal said. “You need one conversation to explain biosimilars in general. In the second round, you can begin to bring in salespeople to explain details. There is really no big education the oncology sales force needs to do beyond explaining why the biosimilar is actually a good product, and why you should be using it, and clearing any questions left from the first round, because there will be counter-detailing from Amgen,” Gal stated.
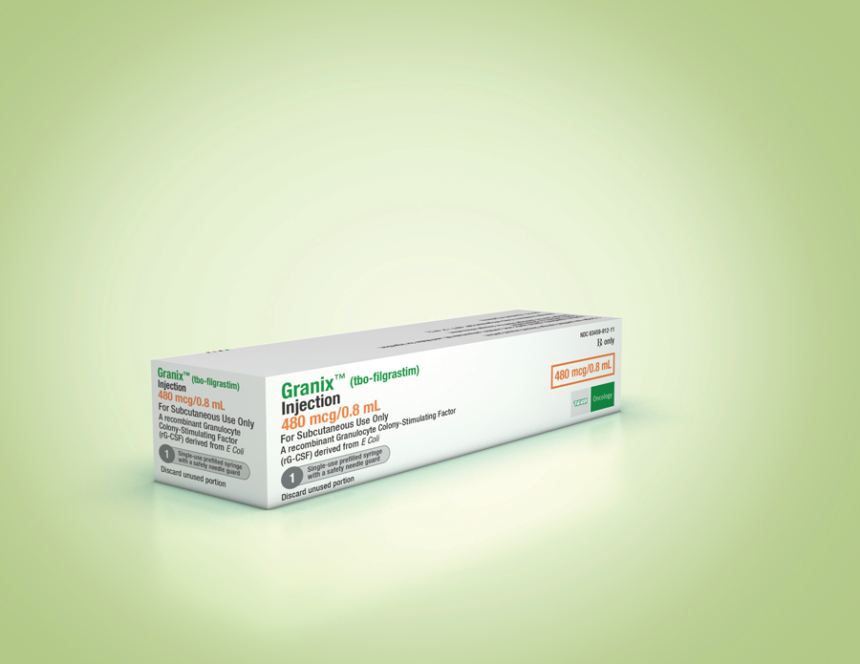
Should it win FDA approval for all five Neupogen indications, as recommended by committee, Sandoz’s filgrastim would launch with an edge over Teva’s Granix, which has been commercially available in the states since 2013, having been approved after clinical studies on its safety and efficacy under the standard 351(a) BLA pathway for biologics, before the biosimilar pathway had been established.
Teva’s filgrastim shares but one indication with Neupogen: reducing severe neutropenia in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs. Teva has also sold Tevagrastim as an EMA-authorized biosimilar of Neupogen in Europe since 2008.
Novartis declined to comment on its marketing plans, or on Zarxio’s cost, to MM&M, but noted that biosimilar list prices are around 20% to 30% lower than that of the originator drugs in the top five EU countries. That’s a range most experts generally forecast for US biosimilars.
Yet filgrastim prices in the US could nose-dive with more filgrastim biosimilars coming to market, despite 40% higher cost to make the drug, says Gal. He predicts Sandoz will come out with a 30% to 35% discount off list price.
“In theory, the gross margin on biologics is in the 95%-plus range,” Gal said. “Biosimilars have room to go way down and still make it profitable. I think we will end up with discounts that are between 30% and 70% after five years in the US.”
In terms of market share, Zarzio’s performance in Europe, where it has surpassed Amgen in units sold, is predictive of US traction, Gal notes.
Since EU launch in 2009, the drug has gained more than 30% share of scripts, making it the first biosimilar to surpass its reference product (Neupogen) and also beating out Chugai Pharma’s Granocyte (lenograstim) to become the most prescribed G-CSF in Europe, Novartis reports.
The Novartis spokesperson said the firm doesn’t break out sales of individual products. The company has reported a 23% increase in biosimilar sales to $420 million in 2013.
“Granix got about 15% share within a year in the US. It’s hard to see Sandoz doing worse,” Gal noted. A low-end estimate has Neupogen competitors taking at least half the US market in five years—80% should other biosimilars join Sandoz’s filgrastim and Granix. “I’m guessing that in five years, Sandoz will be at least half the market.”
Marketers will be waiting for more information on how Zarxio will be characterized, as FDA continues the process of developing standards and guidance for candidates using the 351(k) abbreviated pathway for biosimilars. The agency has announced guidance coming in 2015 on biosimilar labeling, and on naming, such as whether biosimilars can share the same non-proprietary name—in this case filgrastim—with the originator drug.
The FDA is taking up interchangeability, a more stringently defined designation letting pharmacists substitute biosimilars without prescriber approval.
Sandoz has signaled its plans to seek an interchangeable label, but the sales impact for a drug largely administered by hospital doctors could be minimal, Gal pointed out. —David Vaczek
From the February 01, 2015 Issue of MM+M - Medical Marketing and Media