iotech firm Alkermes said Friday it expects Eli Lilly to soon back out of its inhaled insulin development deal between the companies.
“Lilly has informed Alkermes that it is evaluating its business case for AIR Insulin and Alkermes expects Lilly to make a decision to discontinue the program in the next week,” Alkermes said in a news release.
According to Alkermes, Lilly has the right to terminate its license to AIR Insulin at its discretion and expects a Lilly decision based on Lilly’s “evaluation of its own business prospects.”
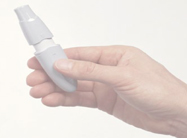
AIR Insulin is a developmental inhalable insulin device that fits in the hand and is being designed to be easy for patients to use. A late-stage trial of AIR was slated to wrap in time for NDA submission of the product in 2009, Bryce Carmine, Lilly’s global brand director, told MM&M in late 2007.
Last October, Pfizer folded its cards on its inhaled insulin delivery device, Exubera, which took a $2.8 billion chunk out of Pfizer’s coffers. Sales of Exubera, knocked for its “bong-like” delivery system, were at just $4 million for the second quarter of 2007.
Following Novo Nordisk’s decision to end development of its AERx iDMS inhaled insulin system in January, biotech firm Mannkind now stands alone as the last remaining inhaled insulin player.
In a statement Monday, MannKind maintained it is “absolutely committed” to the continued development of its Technosphere inhaled insulin product, currently in Phase III testing.
“MannKind recognizes that in order to be successful in today’s healthcare market a product must offer improved efficacy and safety, not just improved convenience,” the biotech firm said. “The decisions of Eli Lilly and Co. as well as Pfizer and Novo Nordisk to discontinue the development of their inhaled insulin products reinforce this view. None of those products offer any advantages over injectable rapid acting insulin analogs.”