Therapeutic Focus: Women’s Health.pdf
From a breast-cancer breakthrough to a polarizing pink pill for hypoactive sexual desire disorder, 2015 has been anything but dull for women’s health. Industry experts point to a host of signs that the category will break free of yesterday’s dusty shackles to recognize conditions and diseases outside female reproductivity.
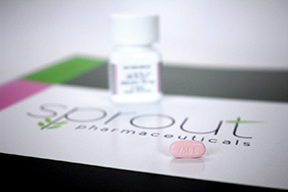
Sprout’s flibanserin shot to headline stardom in June when an FDA advisory committee chose to back the low-libido pill on the condition that it carry safety restrictions to manage side effects. At press time the drug, informally called “female Viagra,” had received FDA approval and Sprout plans to market it as Addyi.
Originally developed as an antidepressant by Boehringer Ingelheim, flibanserin is staring down an otherwise empty female-desire drug category. The drug’s boosters talk about benefits for those who need the option to improve their lives. But those opposed feel that its approval represents a step backward for women’s health.
Negativity about it, according to UC Health Women’s Center director Dr. Lisa Larkin, seems to derive from some who to want to discredit hypoactive sexual desire disorder (HSDD) as a real biologic condition. “The science is clear. I simply can’t understand the magnitude of the controversy,” she says.
Jay Carter, SVP/director of strategy services at AbelsonTaylor, echoes the widely held sentiment that the label “female Viagra” is a poor analogy. He also questions whether the adverse event profile, including fainting, low blood pressure and nausea, will discourage clinicians from acceding to patient requests for the drug.
Prior to Addyi, the lone women’s health drug approval in 2015 was awarded in February to Pfizer’s Ibrance for ER-positive, HER2-negative breast cancer (see Clinical Corner, below). The prevalent cancer type generates a lot of research interest—rightfully so—but other women-specific diseases lack similar pharma investment.
A few forces are converging to resuscitate and expand the women’s health category. A disparity in gender responses to a slew of medications has come face-to-face with a recent emphasis on female clinical trial participation. A general sense of empowerment could finally end the trend of women prioritizing the health of their children, spouses and parents over their own. Natrel Communications’ SVP, director of patient engagement Lisa Hunt, for instance, believes women today are more educated and goal-oriented than ever before, leading them to have more power over their bodies and lives.
Some in the industry are demanding a new definition of women’s health, one that embraces such conditions unique to women as osteoporosis and menopause but that also steps outside to recognize the different ways men and women respond to medications for, say, heart conditions and sleep disorders. Numerous Ambien-fueled daytime drowsiness incidents signaled the need to fine-tune the gender-specific medication pathway. In early 2013 the FDA halved the female dose of the Sanofi insomnia medication, which reverberated as an industry-wide wake-up call. The gender gap exists.
“Back in the day, women’s health meant contraceptives and hormones,” says Phyllis Greenberger, president and CEO of the Society for Women’s Health Research. “We’ve finally convinced the world that women’s health encompasses all products, whether for depression or bone health.”
Hormone vs. non-hormone therapy
Questions continue to surround the widespread use of hormone-replacement therapy, as they have since significant safety issues came to light. Although HRT is still regarded as the gold standard, its declining use has left the door of opportunity wide open for pharma to produce drugs with greater safety profiles.
Greenberger believes misinformation and confusion surround hormone therapy in this country. One exception? When it concerns the presence of the breast-cancer gene in a potential hormone recipient. “If the gene is present, then the hormones may accelerate its presence,” she explains.
CLICK HERE to see the Top 25 Women’s Health Products (in a new tab)
The market now has an expanded array of both hormonal and non-hormonal options for the treatment of vasomotor symptoms in menopausal women. Compounds called selective estrogen receptor modulators, including the approved Tamoxifen, block the stimulating effects of estrogen in breast tissue and may prove stand-ins for HRT.
Noven Therapeutics’ Brisdelle (paroxetine) is the only non-hormonal option the FDA has okayed for the relief of hot flashes, afflicting about half of postmenopausal women. The FDA approved Eli Lilly’s Evista (raloxifene) for the prevention and treatment of postmenopausal osteoporosis, while Pfizer’s Duavee (bazedoxifene-conjugated estrogen) reduces postmenopausal hot flashes and osteoporosis.
In his assessment of the osteoporosis market, Carter believes physicians and payers are looking to reduce spend for the disease, likely for different reasons. “Many options exist for the inexpensive treatment of osteoporosis by generics, both bisphonates and SERMs,” he says. “We’re not seeing a lot of TV advertising for the disease because the opportunity to make money is diminished.”
Nevertheless, biopharma company Radius Health aims to file an NDA and an MAA by the end of 2015 for investigational drug abaloparatide-SC for postmenopausal osteoporosis. Abaloparatide, a new synthetic of a naturally occurring bone-building hormone, is expected to slash the rate of new spinal fractures by a whopping 86%.
Contraceptives evolve
Makers of contraceptives are angling to produce user-friendly and convenient solutions that address the changing needs of a target population with a vast age span. Industry reports indicate an increased worldwide emphasis on category-mainstay oral contraceptives.
OCs are strongly preferred in most markets, Natrel’s Hunt says, because of their convenience, cost and efficacy. Although a shift away from OCs has been challenging, she notes a glimmer of change in the works. New contraceptive methods, including a long-term non-hormonal contraceptive injection in development for men, are needed. Biotech company Evofem has a new drug application for approval of its vaginal gel Amphora as a contraceptive. The non-hormonal gel previously gained FDA approval as a lubricant.
Another major women’s health growth driver has popped up in the most unlikely of places: the intrauterine device market. Belonging to the class of long-acting reversible contraception, IUDs are having a resurgence as an efficacious option for women by eliminating the hassle of taking a daily pill. According to Hunt, today’s women have little to no awareness of the Dalkon Shield controversy, which dates to the 1970s. A new generation of HCPs could help IUD adoption rates with their open-minded approach. “The support of formal recommendations may ease any apprehensions or lingering negative associations with IUD use,” Hunt says.
The US has started to approach contraception in a manner similar to countries in Europe and Asia, where IUDs are a mainstream and preferred contraception option, says Larkin. The new class of IUDs, including Teva Women’s Health’s ParaGard and Bayer HealthCare’s Mirena and Skyla, made room for the category’s newest entrant, Allergan’s Liletta, in February. These devices work by releasing a steady, low level of the hormone levonorgestrel into the uterus.
Getting personal
The women’s health space is also benefiting from industry-wide advances in pharmacogenomics, precision medicine and genetic testing. Companies have developed personalized medicines and molecular diagnostics to address cardiovascular disease and ovarian cancer, and more therapies are in the hunt for approvals for conditions that treat both men and women.
“Sex is the most significant variable and personalized medicine will have to start there,” Greenberger says. “Major pharma is investing in biotechnology and looking at the combo of diagnostics and therapies.” Women tend to suffer milder heart attacks than men and, therefore, cases are going undetected by electrocardiographs and standard tests at a greater rate. A high sensitive troponin test featuring gender-specific thresholds was developed in the UK, aiming to aid diagnosis in women.
Larkin feels drug developers must acknowledge the needs of women and focus on gender-specific drugs as opposed to using male drugs off-label for women. “This is one of the reasons I’m enthusiastic about flibanserin,” she notes.
The Society for Women’s Health Research is beginning to reap the rewards of plans put in motion some 25 years ago to encourage the FDA to require female participation in clinical trials. The NIH agreed in 1993 to include women in Phase-III studies and is currently being encouraged to look at Phase I and II because of the important toxicity and safety concerns addressed.
“We are finally seeing change, but it takes time for change to be fully implemented and for researchers to figure out what they need to be doing,” Greenberger says. In response to industry concern, the FDA began including clinical trial snapshots to encourage pharma to follow suit by representing sex and ethnic differences.
The real issue with trials is not about inclusion, but rather data analysis. “The question is, are there enough women in the trial to determine whether the drug is clinically relevant,” Greenberger continues. “Pharma knows it needs to include women, but companies don’t analyze the data by sex. If they only look at the bottom line, then they won’t see the gender differences.”

Pfizer’s Ibrance has broken through clinical and scientific barriers to open up a new breast-cancer treatment avenue. The CDK 4/6 inhibitor has the potential to impact the way oncologists treat postmenopausal women with estrogen-receptor-positive human-epidermal growth-factor-receptor-2-negative (ER+/HER2-) advanced breast cancer, who comprise about 60% of all women with advanced breast cancer.
As the second-most-common type of cancer in the US, breast cancer claimed 40,000 lives in 2014, the National Cancer Institute reports. Celebrated as the first significant advancement for women in the first-line setting in nearly 10 years, Ibrance gained FDA approval in combination with letrozole as initial endocrine-based therapy for metastatic disease. Ibrance also earned the distinction of receiving the first FDA nod out of its class of anti-cancer agents that specifically target CDK 4/6.
By gaining the FDA’s blessing two months ahead of schedule, Ibrance hit the ground running on launch day. The drug raked in $38 million during its first quarter on the market, exceeding analysts’ predictions by a few million. Some of those analysts say that the early approval, combined with the drug’s breakthrough therapy designation and priority review, could help Pfizer reach $4 billion in sales in the next five years.
 According to Dr. Julia Perkins Smith (photo, left), Pfizer Oncology’s senior medical director, oncology remains a major focus in women’s health. “Targeted therapy uses drugs or other substances to more precisely identify and attack cancer cells,” she explains. “There is extensive research in this area, with the hope of improving outcomes for a variety of cancer patients, including women with breast cancer.”
According to Dr. Julia Perkins Smith (photo, left), Pfizer Oncology’s senior medical director, oncology remains a major focus in women’s health. “Targeted therapy uses drugs or other substances to more precisely identify and attack cancer cells,” she explains. “There is extensive research in this area, with the hope of improving outcomes for a variety of cancer patients, including women with breast cancer.”
In the Phase II PALOMA-1 trial, the data on which the drug was approved by the FDA, women treated with Ibrance (palbociclib) plus letrozole showed a substantial improvement in progression-free survival or PFS (median PFS of 20.2 months) compared with women who received letrozole alone (median PFS of 10.2 months), Smith says.
Pfizer is conducting two Phase-III trials in women with HR+/HER2-metastatic disease. PALOMA-2 is a Phase-III study that evaluates palbociclib in combination with letrozole versus letrozole plus placebo as a first-line treatment for postmenopausal patients with ER+/HER2- advanced breast cancer. PALOMA-3 is a Phase-III study that evaluates palbociclib in combination with fulvestrant versus fulvestrant plus placebo in women with HR+/HER2- metastatic breast cancer whose disease has progressed after prior endocrine therapy.
“Pfizer has worked closely with experts to establish a robust development program for palbociclib in ER+, HER2 breast cancer across stages and treatment settings as well in other tumor types,” Smith says.
Up next? In the fourth quarter of 2015 Pfizer plans to submit a supplemental New Drug Application to the FDA for palbociclib in combination with endocrine therapy for women with HR+/HER2-metastatic breast cancer whose disease has progressed during or after endocrine therapy based on the results of the PALOMA-3 study.
From the September 01, 2015 Issue of MM+M - Medical Marketing and Media