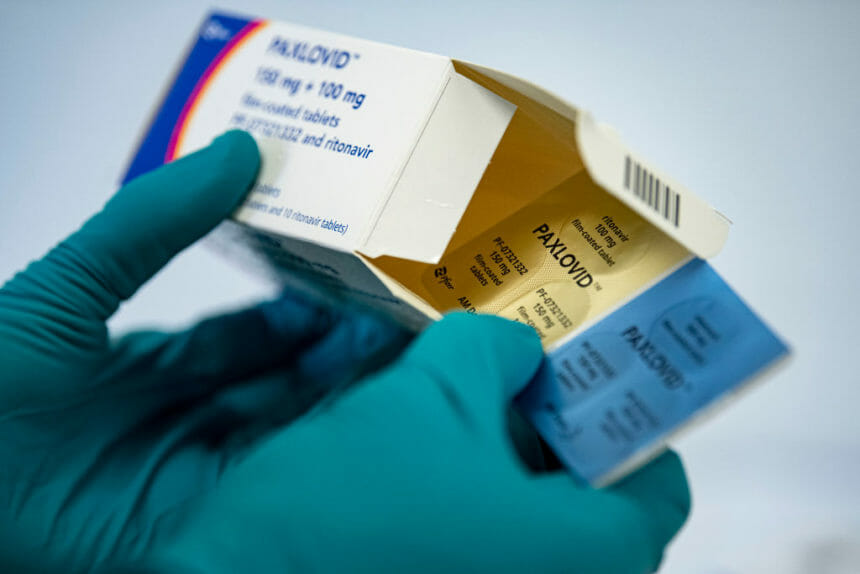
The Food and Drug Administration has granted full approval for Pfizer’s oral COVID-19 pill Paxlovid. The U.S. government had purchased a number of courses of the drug to be available for free to the public, but the drug will move to a commercial market when that inventory runs out. (Reuters)
Shareholders of biotech company Illumina have ousted the board chairman John Thompson following a two-month proxy battle between the company and investor Carl Icahn. Icahn had previously criticized Illumina’s acquisition of Grail, saying the move was “reckless,” and urged shareholders to oust Thompson as well as CEO Francis deSouza. (CNBC)
Noom, a startup that for years has touted a psychological path to weight loss, said it will launch an option that will include prescriptions for obesity drugs such as Novo Nordisk’s Wegovy for about $120 a month. It’s the latest weight-loss company to join the lifestyle-focused industry’s push into using highly effective, costly GLP-1 obesity drugs to help customers slim down. (Bloomberg)
AbCellera has received a $515.9 million investment from the government of Canada and the province of British Columbia to allow the company to boost infrastructure around its antibody therapies. AbCellera plans to build a biotech campus, where it will start 17 drug development programs focused on immune diseases and cancer. (Endpoints News)
Bristol Myers, J&J’s blood clot drug milvexian received a fast track designation from the FDA. The tag includes all three indications under evaluation in the Phase 3 Librexia program: ischemic stroke, acute coronary syndrome and atrial fibrillation (Seeking Alpha)