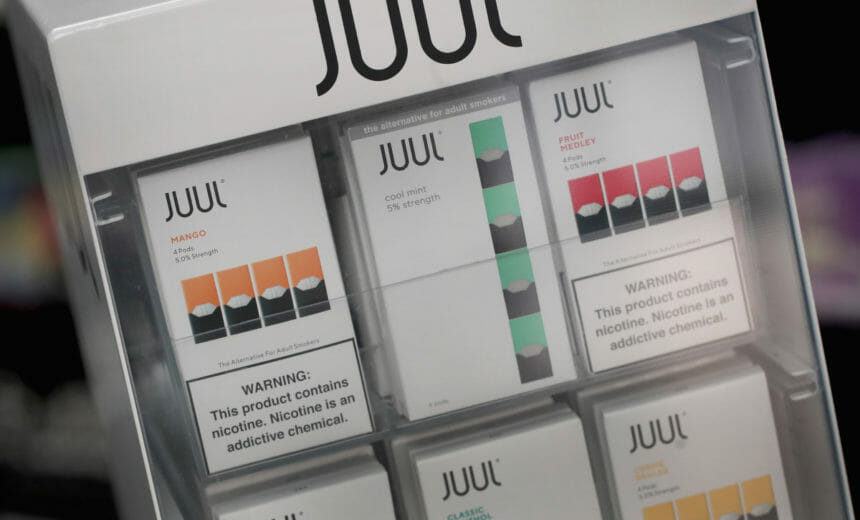
The House Oversight Committee has called out Juul for spurring the teen vaping epidemic during the panel’s first day of a two-part hearing on the company’s marketing tactics and health claims. Committee Chairman Rep. Raja Krishnamoorthi (D-IL) said that he wants Juul to offer more satisfactory answers to help find a solution to the rise of teen vaping. (CNBC)
Wearable devices are unable to reliably track the heartbeats of people of color. Devices from Fitbit, Garmin, Samsung and others reportedly use problematic technology in their trackers that includes lights that are not able to accurately read skin with more melanin. The issues are likely to affect scientific research done through wearables. (STAT)
The Food and Drug Administration has asked Allergan to recall its textured breast implants after discovering a link between the product and cancer. Analysis showed that the implant was six times riskier than similar products. Thirty-eight countries have recalled the implants. (NBC)
Cardinal Health counsel Jennifer Norris has stated the drug distributor is not obligated to publicly share the amount of opioids it has shipped, according to court documents made public this week. She also said that she is uncertain whether the company has a duty to the public to attempt to prevent harm when it comes to its prescription opioids. The trial involving these documents is set to begin in October. (Associated Press)
The Senate Finance Committee has introduced a drug-pricing bill that includes 30 amendments to Medicare and Medicaid. Notable changes include capping both out-of-pocket costs for seniors on Medicare and price increases for drugs available on both Medicare and Medicaid. (MM&M)