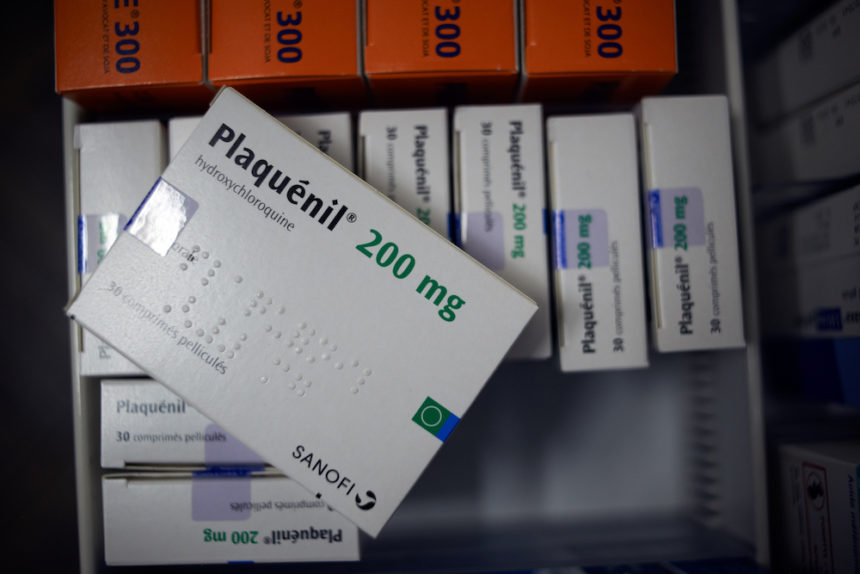
The Food and Drug Administration has revoked emergency use for hydroxychloroquine in COVID-19 patients. After the FDA reviewed studies of the drug, it found the risks did not outweigh benefits and was “unlikely to be effective.” The antimalarial drug was touted by President Donald Trump. (The New York Times)
Eli Lilly began a trial of its rheumatoid arthritis drug to treat coronavirus patients. The drug, Olumiant, will be tested in 400 hospitalized COVID-19 patients globally. It is being tested to see if it can reduce deaths from the COVID-19 illness and lessen its severity. (Reuters)
The FDA has approved a video game to treat children with ADHD. This is the first time a treatment like this has been approved. The game, called EndeavorRx and made by Akili Interactive Labs, can help children improve attention function. (STAT)
A health official has warned that states may have to reimplement safety measures if coronavirus cases spike. An infectious disease official from the Centers for Disease Control and Prevention said the decision to put social distancing or stay-home measures will be made locally. (CNBC)
AstraZeneca has signed supply deals for its potential COVID-19 vaccine. The company, which is developing the vaccine with Oxford University, signed deals with drug manufacturer Catalent Biologics and the Inclusive Vaccines Alliance, a consortium of four European countries. (BioPharma Dive)