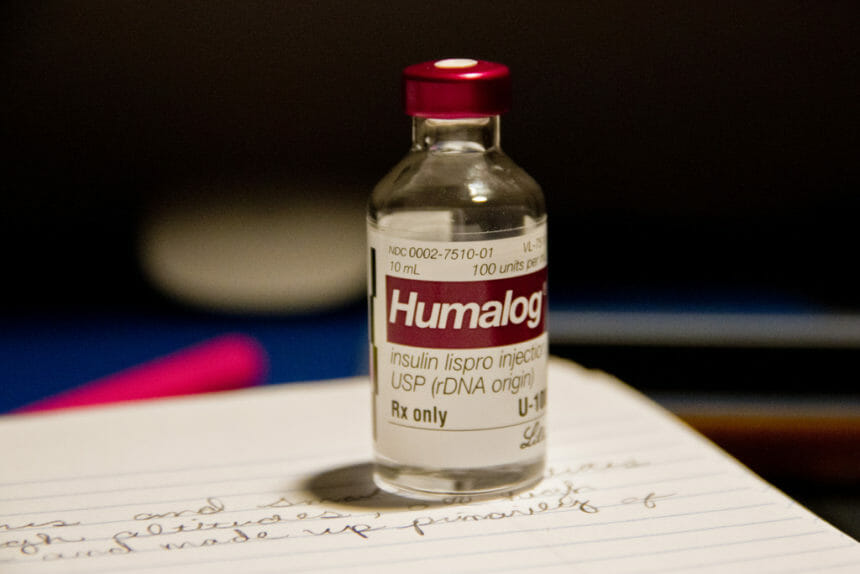
Eli Lilly has revealed the confidential pricing structure for blockbuster insulin injection Humalog, showing the discrepancy between what the company charges wholesalers and what patients pay. (CNBC) The drugmaker has been facing political pressure over the price of insulin. (Endpoints)
The FDA has assigned breakthrough designation for the first time to a device for patients with heart failure. The Optimizer Smart System can help patients in advanced stages of the disease by making the heart squeeze more effectively. (ABC News)
Oklahoma’s supreme court has denied a request from drugmakers to postpone the beginning of lawsuits accusing the companies of fueling the opioid epidemic. The state’s attorney general sued 13 opioid manufacturers in 2017, claiming they engaged in marketing pushes that resulted in scores of overdoses and deaths. (Associated Press) Update: Purdue Pharma settled with the state on Tuesday morning (MM&M).
Biogen said early Monday that it plans to buy back $5 billion in shares. The announcement came days after it said it would end Alzheimer’s disease trials, cutting $18 billion from the company’s value last week. Biogen’s board authorized a previous stock-buyback program last August. (CNBC)
Bayer and Johnson & Johnson have agreed to settle more than 25,000 lawsuits over their Xarelto blood thinner for $775 million. The two companies will split the compensation amount equally. Neither company has to admit liability under the agreement. (Reuters)