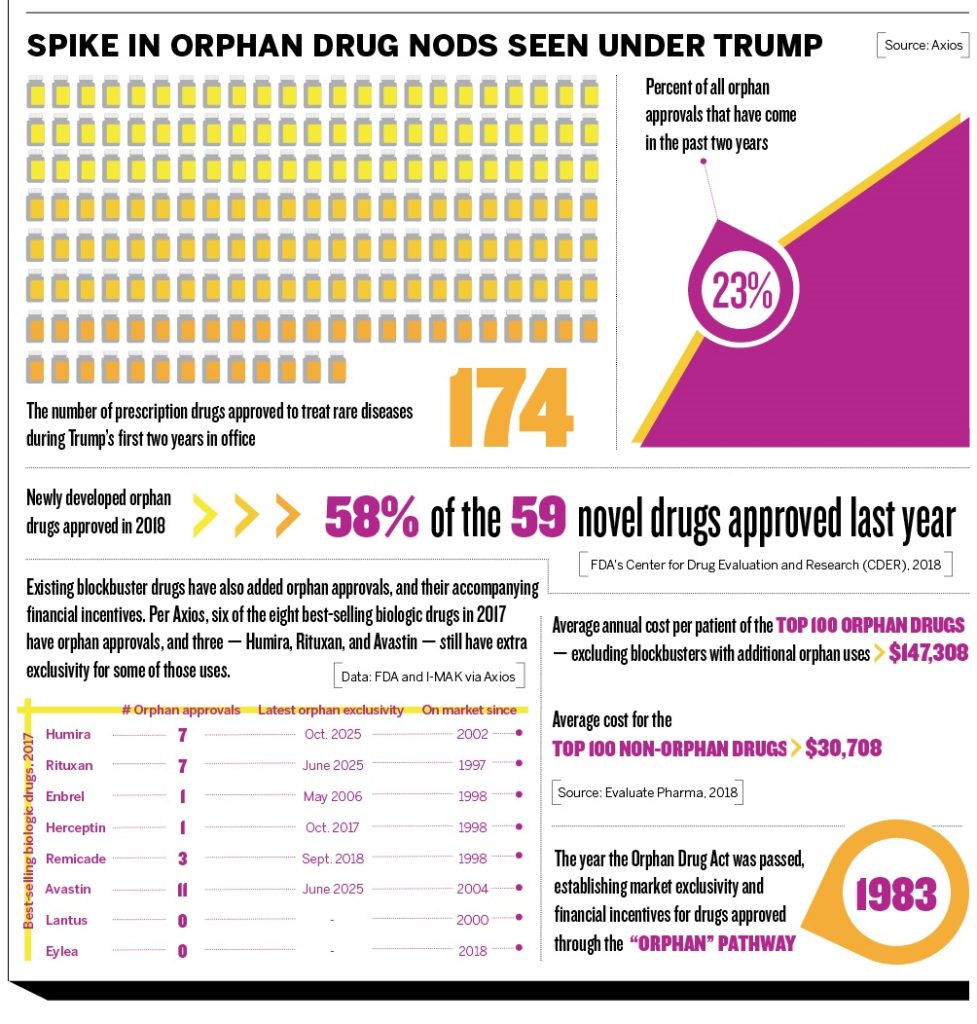
Last year, the FDA gave the green light to 59 novel orphan drugs, the most it has approved in nearly two decades. The rate of approvals has escalated during the first two years of the Trump administration. However, the Orphan Drug Act continues to take heat for letting manufacturers maintain high prices during an exclusive selling time and for giving orphan drug designations to existing treatments. Here’s a closer look at orphan approvals in 2018.
From the April 01, 2019 Issue of MM+M - Medical Marketing and Media






