Novartis is scaling up R&D for a targeted compound based on promising early-stage evidence the agent benefits lung-cancer patients, including those whose tumors progressed after taking another personalized medicine.
“A robust clinical development plan is underway” for LDK378, the Swiss firm said yesterday. It anticipates a regulatory submission by early next year, although a spokesperson told MM&M that Novartis hasn’t specified the location of the first filing. It’s also readying an unspecified number of Phase III trials to make sure results seen in Phase I are supported in a larger group. Two Phase II trials are already happening.
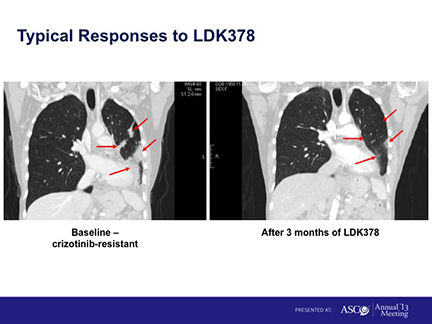
The drug is designed for a subset of non-small cell lung cancer (NSCLC) patients whose tumors are positive for a genetic mutation called anaplastic lymphoma kinase, or ALK. According to an update of the Phase I results, presented at the 2013 ASCO meeting, overall, tumors in 47, or 60%, of the ALK+ NSCLC patients taking the highest dose responded to the drug. Tumors hadn’t progressed for a median of 8.6 months.
The study included both patients who had taken crizotinib and those who hadn’t, crizotinib being the Pfizer drug for ALK+ NSCLC patients sold under the brand name Xalkori.
That’s important because many patients relapse after taking crizotinib. “You can control the tumor for a finite amount of time, and that time is measured in months not in years,” said Richard Wagner, PhD, a VP with Kantar Health. “In ALK, it looks like you could use the Novartis drug and get what seems to be another eight months [before the tumor progresses again], which is not bad.”
The response to LDK378 was slightly higher, 62%, in crizotinib-naïve patients. Novartis plans to enroll more than 1,100 patients with ALK+ NSCLC at sites worldwide for its Phase III trials.
“We’re encouraged by the data, and that’s why we’re developing a pretty robust plan around it, to make sure the Phase I data [are] supported,” the spokesperson said.
Novartis’ decision to bring LDK378 into Phase III comes after the FDA assigned the compound its “breakthrough therapy” designation back in March. It’s awarded based on preliminary clinical evidence the drug could substantially improve treatment for serious or life-threatening diseases. NSCLC is the most common type of lung cancer, accounting for 85-90% of all cases, but those with the ALK+ mutation are a much smaller subset, around 5% of NSCLC.
Close behind Pfizer and Novartis for the ALK+ NSCLC market is Roche, which has presented promising data in crizotinib-naïve patients. Roche also plans to take its own drug, CH5424802/RO5424802, into Phase III. Noted Wagner, “We can see a time when that population is split between three or more drugs and companies.”