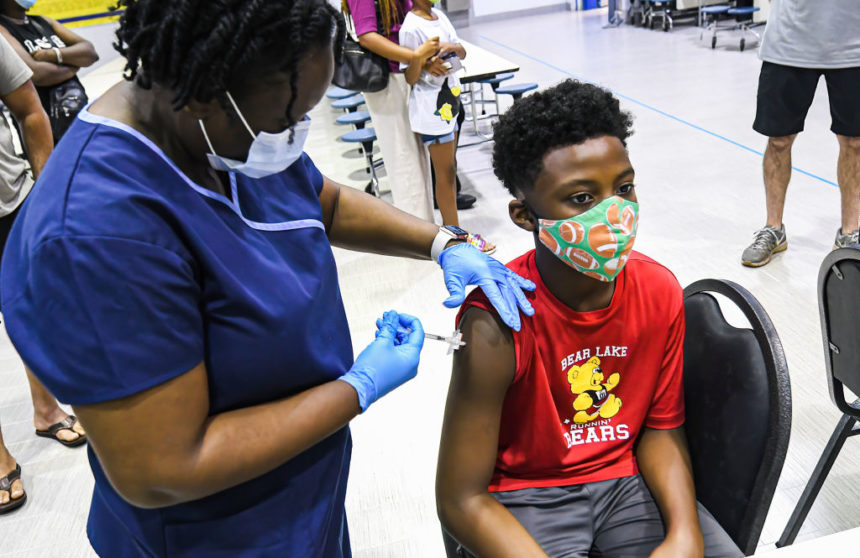
The Pfizer/BioNTech COVID-19 vaccine is safe for children aged five to 11, the companies announced. The drugmakers plan to submit the data to the Food and Drug Administration (FDA) “as soon as possible,” they said. (CNBC Weekly)
U.S. health officials including Dr. Anthony Fauci said they support the Food and Drug Administration advisory panel’s recommendation for booster shots to be limited to people over the age of 65. Fauci noted that booster shots are not yet necessary for the general population. (Politico)
Mirati Therapeutics has added David Meek to its leadership team as CEO, replacing Charles Baum. Meek previously led gene therapy company FerGene and has held leadership positions at Ipsen, Baxalta and Novartis. (Reuters)
Advocates for drug pricing regulation are scrambling to fight back against moderate Democrats who formally oppose their party’s pricing package. Advocacy organizations are launching ads, mobile billboards and advertising campaigns to support drug pricing policy. (STAT)
Exelixis’ thyroid cancer drug Cabometyx showed promise in its Cosmic-311 clinical trial. Differentiated thyroid cancer patients treated with the drug lived a median of 11 months without the disease getting worse, compared to 1.9 months for patients on placebo. (Endpoints News)