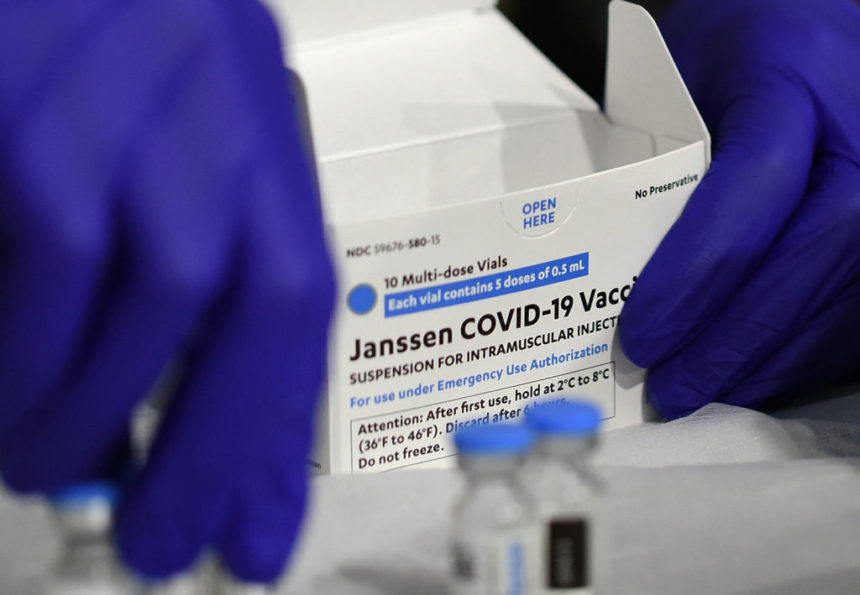
After six reports of rare blood clots in women, U.S. health regulators advised temporarily halting administration of the Johnson & Johnson COVID-19 vaccine. Some 6.8 million Americans have received the vaccine with no reported issues. (The New York Times)
The Food and Drug Administration is lifting restrictions on delivering abortion pills via mail during the pandemic. It reverses a policy put in place by the Trump administration. (Politico)
Spark Therapeutics has confirmed a $645 million deal with Senti Bio. Spark plans to harness Senti’s synthetic gene circuit tech to focus on programs for the central nervous system, eye and liver. (Endpoints News)
COVID-19 cases are increasing in more than half of U.S. states, with a seven-day average of 69,000 daily new cases. But the pace of vaccinations is also rising, with more than one in five Americans now fully vaccinated. (CNBC Weekly)
Setbacks within the last month in developing treatments for Huntington’s disease have left patients disappointed. But researchers hope to learn from the failed trials, including studies of therapies from Roche and Wave Life Sciences. (STAT)