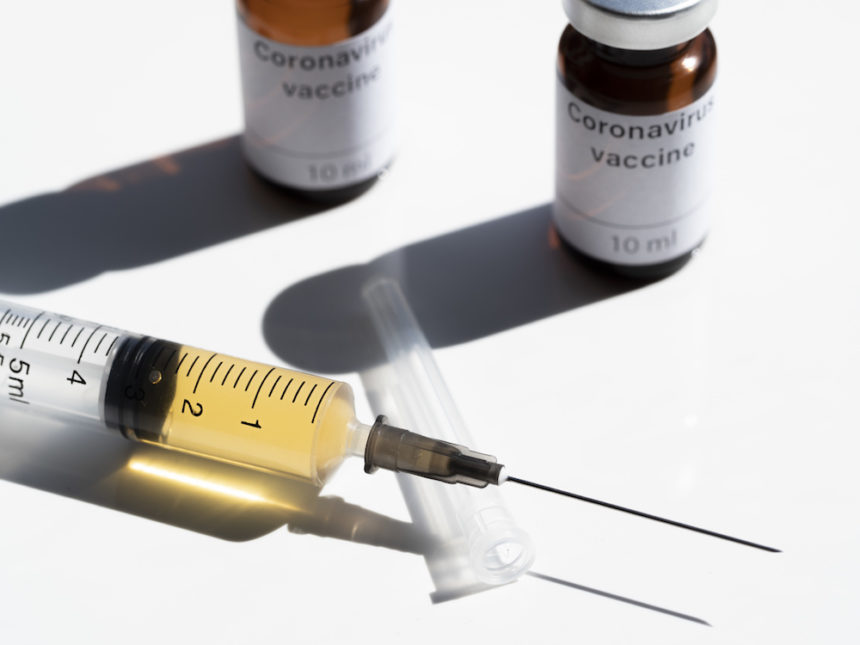
Nine pharma companies pledged to only seek approval for COVID-19 vaccines proven to be safe and effective. The industry is attempting to persuade the public that the vaccine approval process is not being influenced by politics. The group includes companies currently leading the vaccine race: Moderna, Pfizer and BioNTech and AstraZeneca. (STAT)
A coronavirus vaccine before the election is unlikely, said Dr. Anthony Fauci, White House coronavirus adviser. He said it’s more likely to be ready by the end of the year, despite President Trump claiming it could be ready by November. (CNBC)
Uber Health head Dan Trigub to depart. Trigub is leaving Uber to start his own healthcare company. He has led Uber Health for the past two years as the company built its medical transportation service. (STAT)
Leaders at the Food and Drug Administration are insulating employees from political pressure. The agency leaders said they will abide by the June guidelines that set the standard for a potential vaccine’s approval and told all employees that the review will stick to the science. (Bloomberg)
Blueprint Medicines and Roche won approval for its lung cancer drug from the Food and Drug Administration. The drug, Gavreto, will treat a small subset of lung cancer patients with certain gene abnormalities. The treatment will cost $19,250 per month. (BioPharma Dive)