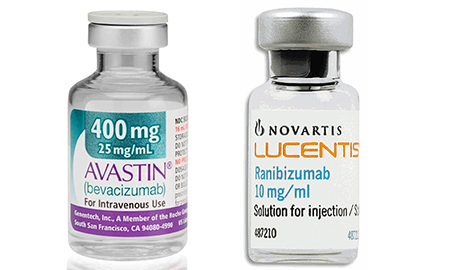
A regional court in Italy is supporting a government agency’s allegations that Novartis and Roche colluded to give Lucentis a marketing advantage over Avastin. The drugmakers tell Reuters they plan to appeal the decision. The anti-trust agency fined the two companies $226 million in March, and Italy’s Health ministry also plans to seek damages. Italy is not the only place where the companies are being criticized over these very same drugs. A 2013 Washington Post piece discussed a marketing strategy that gave Lucentis an advantage and researchers reported this summer that swapping Avastin for Lucentis in some eye-related conditions could save the US about $18 billion. The catch: Avastin is indicated for cancer treatments and the drugmakers are not pursuing eye indications. The companies have argued that replacing Avastin in Lucentis prescriptions is risky because Avastin has not been tested for ocular use.
AstraZeneca and Ranbaxy are the first drug companies to test out the new US ruling that drugmakers can be sued for pay-for-delay deals. Bloomberg reports losing could cost AstraZeneca billions in damages. The lawsuit’s foundation is a 2008 patent settlement which kept generic Nexium off the market, essentially extending the branded drug’s patent life beyond May 2014.
President Barack Obama is urging Congress to fund Ebola eradication research. The New York Times reports that the president urged lawmakers to put $2.6 billion in emergency funding towards research Tuesday when speaking at the National Institutes of Health. “We cannot just fight this epidemic . . . we have to extinguish it” he said. No new cases have been declared in the US but concerns persists: the Boston Globe says a patient is being tested for the virus at Massachusetts General Hospital, and the United Nations is not going to hit its containment targets: Bloomberg reported Monday that Ebola cases are surging in Sierra Leone, even as they decline in Liberia and Guinea.
Biogen Idec said it is planning a Phase-III trial of the treatment for its experimental Alzheimer’s drug BIIB037, Reuters reports. Tested in patients with mild symptoms of the disease, or in patients that had possible signs of Alzheimer’s, the drug in the early stage study cut brain plaque levels and significantly improved cognition. The most significant side effects seen with BIIB037 were signs of possible brain swelling and tiny hemorrhages, which the company described as “largely mild to moderate, and self-resolving,” the company reported.
FDA has issued a final rule that sets standards for labeling of prescription drugs and biologic medicines used during pregnancy and breast feeding. The new label content and formatting requirements replace an “overly simplistic” letter system that has been used to classify the drugs which has been misinterpreted as a grading system, FDA said. Providing a more consistent way to include relevant information, the new labeling rule provides for explanations on risk and benefits. It requires the use of three detailed subsections titled “Pregnancy,” “Lactation” and “Females and Males of Reproductive Potential” that provide details on use of the product and describe risks “within the real-world context of caring for pregnant women who may need medication,” FDA said. The agency has issued draft guidance to help drug and biological product manufacturers comply with the new labeling content and format requirements.