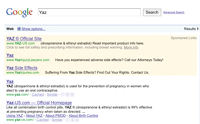
Bayer’s Yaz is trying out a paid search format similar to that proposed by Google for products carrying black-box warnings.
The Yaz-branded sponsored link appears in a yellow-highlighted box atop Google searches on terms including “Yaz,” “the pill” and “birth control.” It features a headline linking to the product site, a line of text advising viewers to “Read important product info here,” and a bottom line reading “Click to see full safety and prescribing information, including boxed warning,” followed by a “fixed” link to physician labeling.
Yaz carries a boxed warning of sharply increased cardiovascular risks for users who smoke, particularly those over the age of 35. Boxed warnings denote particularly serious risks and prescription drug brands carrying boxed warnings are not allowed to run so-called reminder ads giving a brand name but not its indication.
Bayer was among the 14 recipients of untitled letters for violative sponsored links issued by FDA’s Division of Drug Marketing, Advertising and Communications in April. Drug companies had been operating under the so-called “One click rule,” the assumption that as long as full-risk information was a click away, they could run space-limited sponsored links giving only a name and abbreviated indication. In DDMAC’s letter to Bayer, which concerned sponsored links for Yaz as well as Levitra and Mirena, the agency said: “omission of risk information is particularly concerning as one of these products, Yaz, has a boxed warning.”
The agency’s assertion that companies could not give a product’s approved use in a sponsored link without listing full-risk information prompted pharma paid search rates to plummet 84% by the end of June, according to comScore data.
At FDA’s hearings last week on promotion of prescription drugs using the internet and social media, Google’s Mary Ann Belliveau and Amy Cowan argued that the agency’s actions on sponsored links had made them less relevant and transparent, noting that clickthrough rates had nosedived since April.
Google proposed a new format featuring: a headline linked to a designated landing page, usually the product site homepage; a line of text featuring chemical name and, for those not carrying black-box warnings, a short description of the product’s intended use; and a second line of copy listing major risks that is fixed and cannot be modified, followed by a link to full-risk information.